Identify Relevant Regulations, Standards, and Laws
Once again, AHIMA steps up and provides a thorough source to guide the HIM professional on a host of documentation issues. Some of this information leads into next week’s content regarding storage of sensitive PHI.
The guidance for designated records is particularly timely as health technology expands the types of records flowing into the PHI data set. Digital x-rays, scans, and other diagnostics, interoperability that provides for a host of consult dictation becoming part of the record, home monitoring, telemetry monitoring, and a myriad of other documents must be addressed. Should they be included or is a narrative sufficient? If included where are they stored? How long are they stored? What exactly does the EHR include?
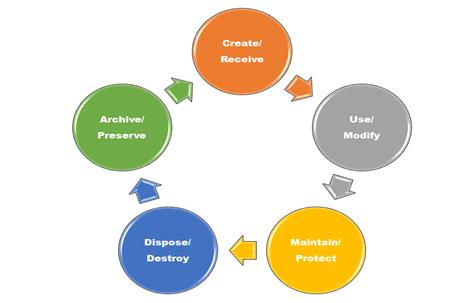 Photo Credit: https://www.smartsheet.com/medical-records-management
Photo Credit: https://www.smartsheet.com/medical-records-management
For years healthcare organizations have struggled to define their legal health records and align them with the designated record set required by the HIPAA privacy rule. Questions often arise about the differences between the two sets because both identify information that must be disclosed upon request.
The expanding scope of health records adds to the challenge of defining and compiling these record sets. An individual’s record can consist of a facility’s record, outpatient diagnostic test results or therapies, pharmacy records, physician records, other care providers’ records, and the patient’s own personal health record. Administrative and financial documents and data may be intermingled with clinical data.
In addition, the type of media on which information is recorded is also expanding. Source records may include diagnostic images, video, voice files, and e-mail. The organization must determine which of these data elements, electronic-structured documents, images, audio files, and video files to include.
The emergence of electronic health records (EHRs) also is complicating organizational efforts to define and disclose information. Information in EHRs is often stored in multiple systems, inhibiting the ability to succinctly pull together the record for either the legal health record or the designated record set.
These input systems may include laboratory information, pharmacy information, picture archiving and communications, cardiology information, results reporting, computerized provider order entry, nurse care planning, transcription, document imaging, and fetal trace monitoring systems, as well as a myriad of home-grown or individual clinical department systems.
However, the same criteria that organizations used to determine what paper records to retain and include in their legal health records and designated record sets can be applied to electronic records. Questions organizations must ask include:
What information can be stored long term?
What is clinically useful long term?
What is the cost of storage?
How can the organization effectively and succinctly assemble the EHR for long-term use?
This practice brief compiles and updates guidance from four previously published practice briefs to provide an overview of the purposes of the designated record set and the legal health record and helps organizations identify what information to include in each. It also provides guidelines for disclosing health records from the sets. The four original practice briefs are listed in the “Sources” section at the end of this practice brief.
Fundamentals of the Legal Health Record and Designated Record Set
Provide a plan for guidance for defining of electronic record health sets based upon AHIMA standards.
Include each of the following aspects in the assignment:
Ø Access the AHIMA document: Fundamentals of the Legal Health Record and Designated Record Set
Ø Create a chart with columns to place each of the five criteria to evaluate when defining what is part of the HER
Ø Locate the five criteria in the middle of the document
1. Identify Relevant Regulations, Standards, and Laws
2. Determine Records Created in the Course of Business
3. Address Retention Requirements
4. Consider How Data Would Be Produced
5. Classify External Records
Ø Place each criterion in a column and list how you will define each
Ø Submit your Word document chart to the drop-box
Ø Cite the AHIIMA reference. APA is not required but as always proper grammar, sentence structure and spelling is expected. There is NO copy/pasting. Everything must be in your own words.






